Qualis LIMS Laboratory Workflow
1
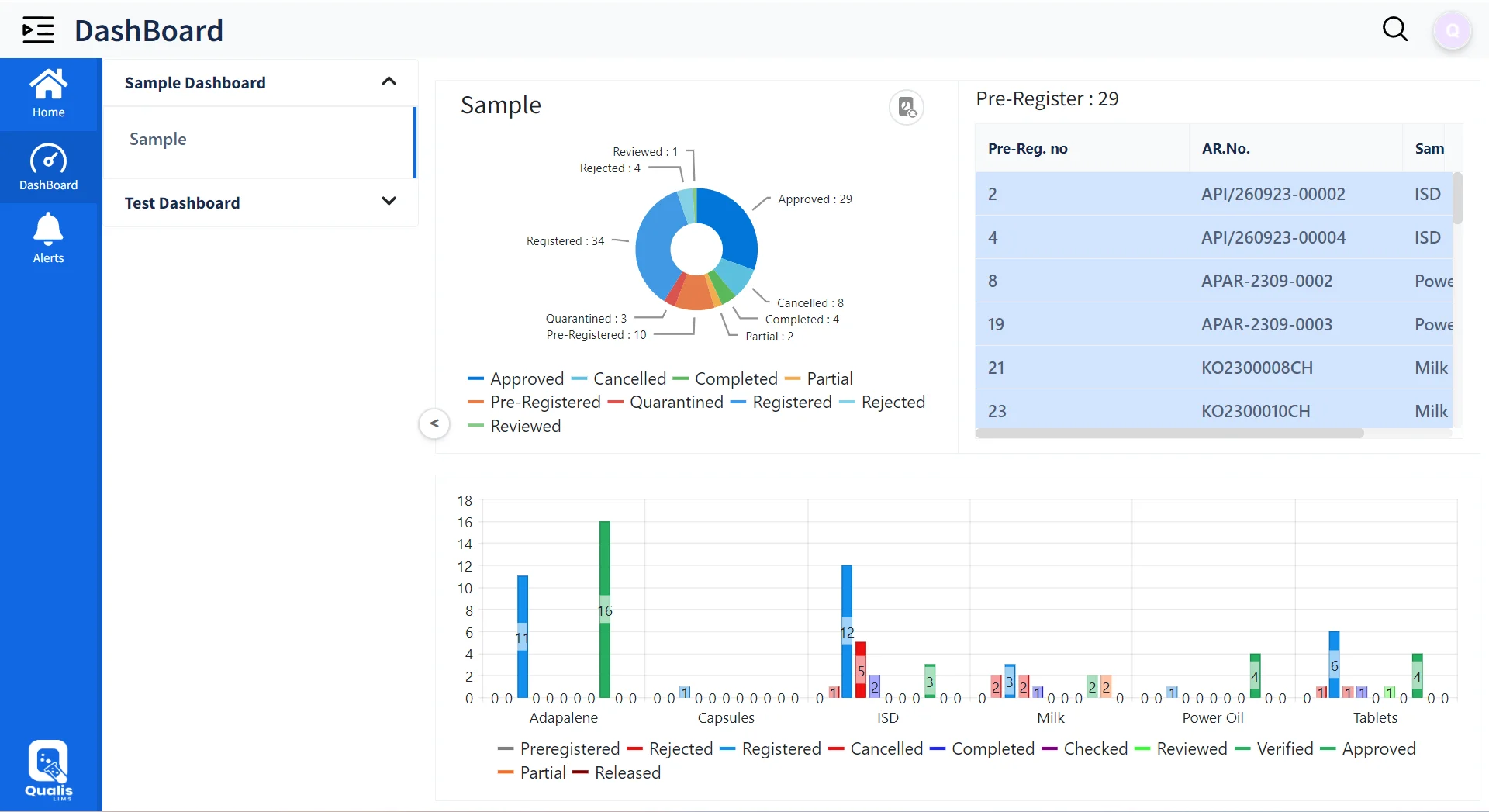
Sample Pre-Registration/ Registration
2
Result Entry (Manual, Instrument interface)
3
Approval & Release (HOD, Study Director, CRO)
4
Reports
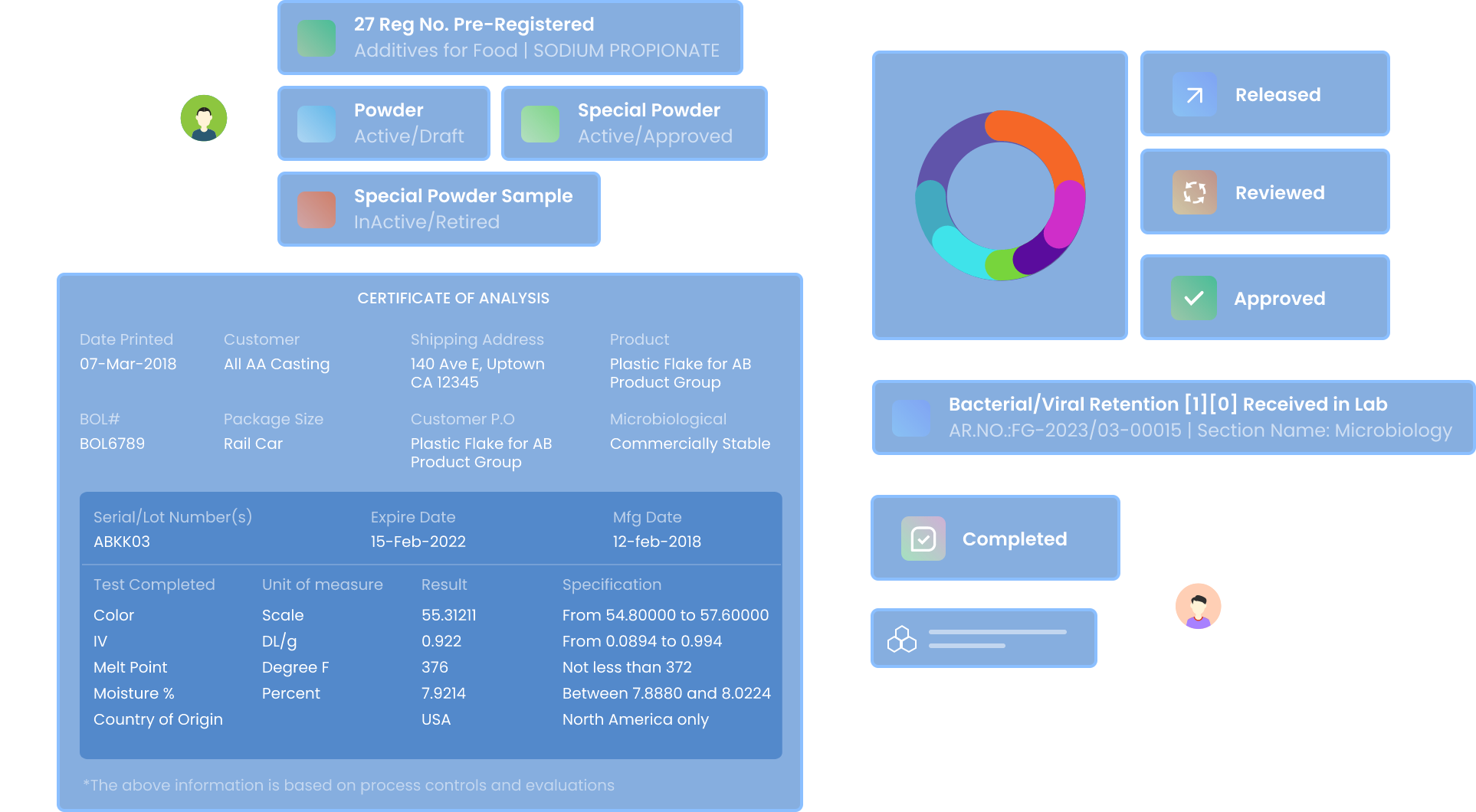
Design your Input templates for Dynamic Sample Registration
Design dynamic templates to register different samples received in the lab. Keep track of the sample meta-data throughout its lifecycle.
Design
Drag and Drop various input fields such as Number, Text, Date etc. to design dynamic templates for each sample type.
Validate
Validate and approve templates and link them to various tests. Modify existing templates with version and release control.
Register
Register Samples for different types of Tests using pre-validated templates for your day-to-day activities
Learn how Qualis LIMS can fit your Small, Mid-size or Enterprise Scale Lab today!
Qualis LIMS functional Highlights
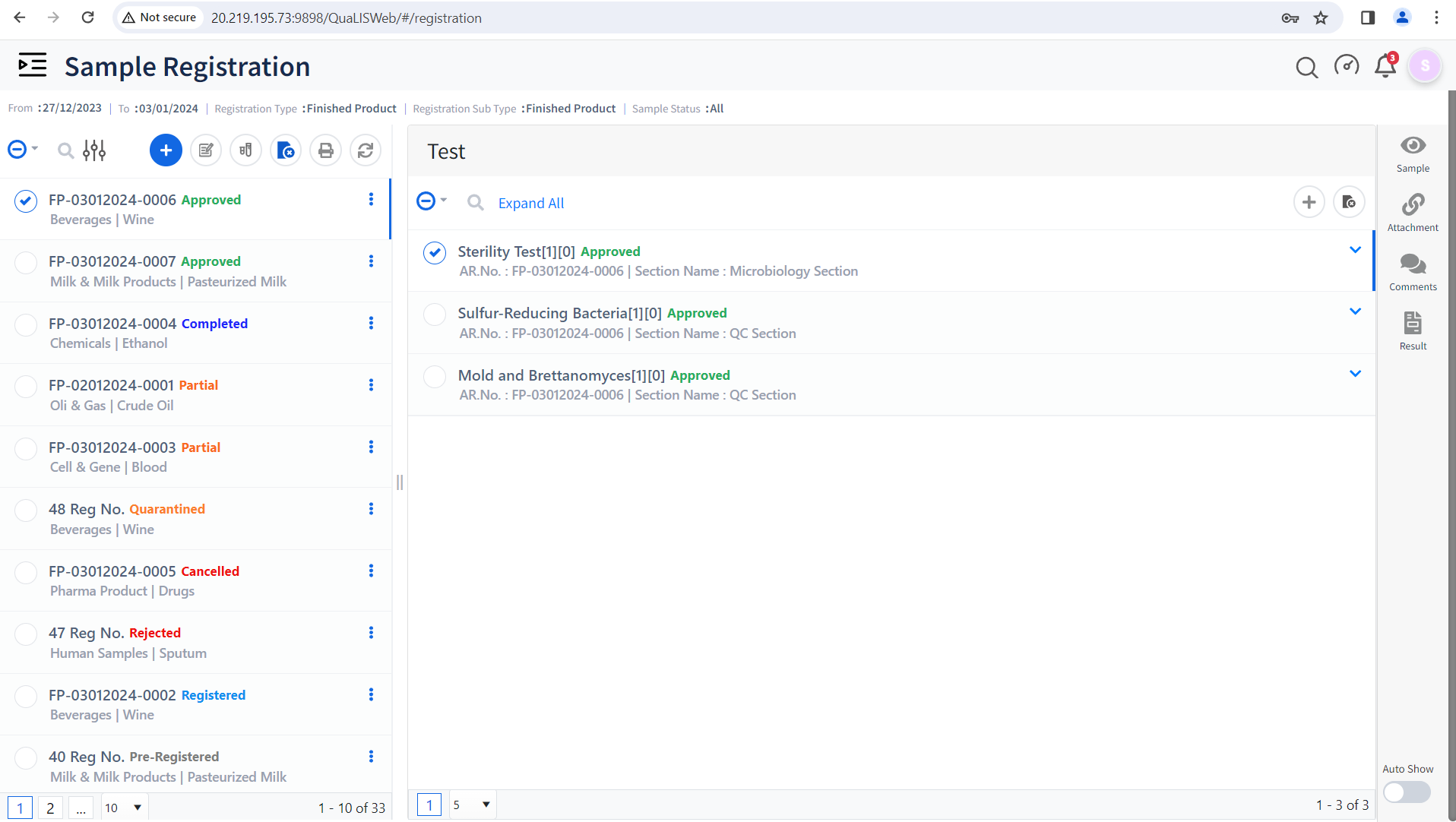
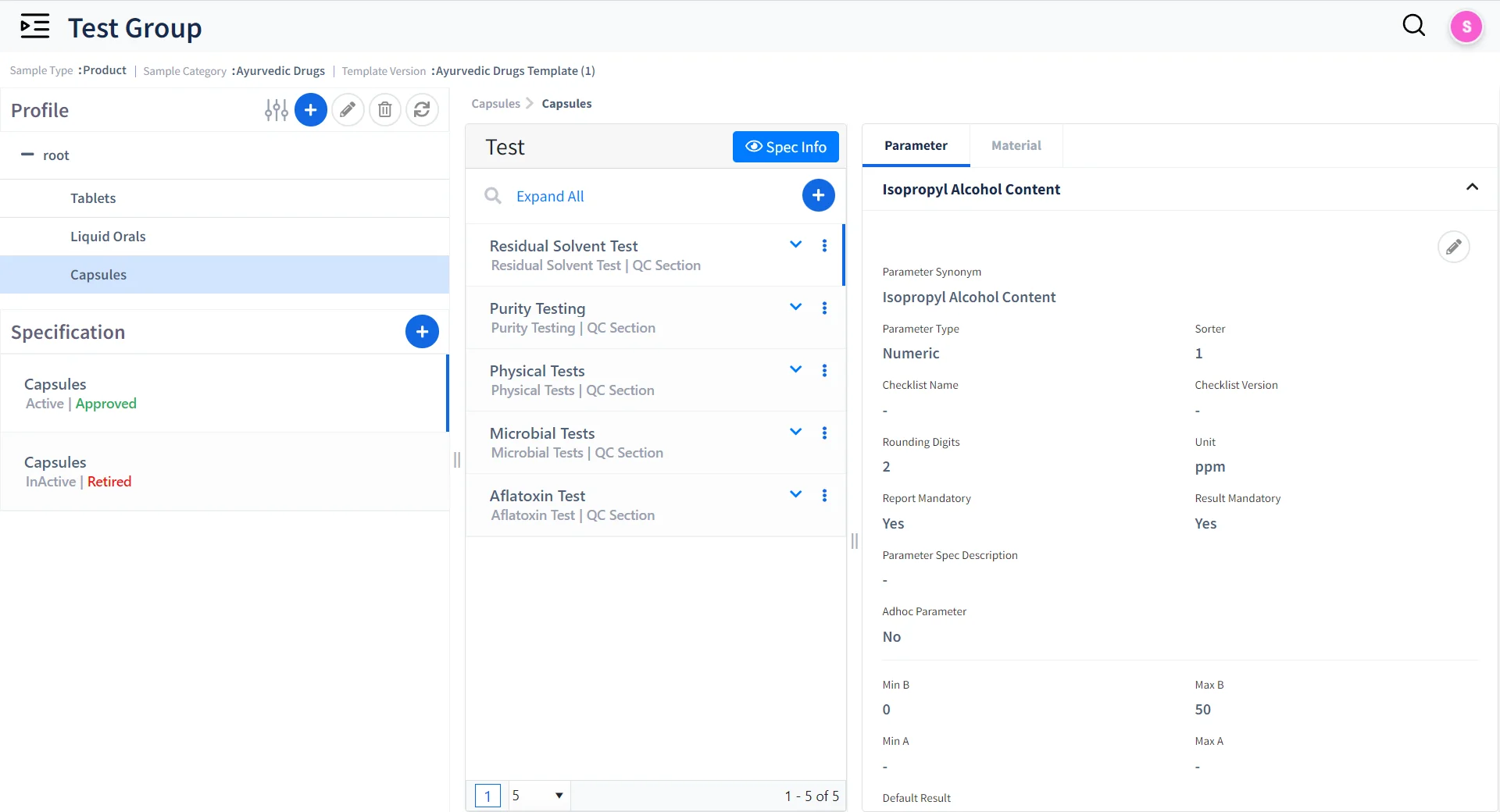
Unified Test and Specification Management
- Create and manage multiple test groups for samples based on Test Parameters.
- Manage test specifications with Version control and release control.
- Set Limits based on expected test Results
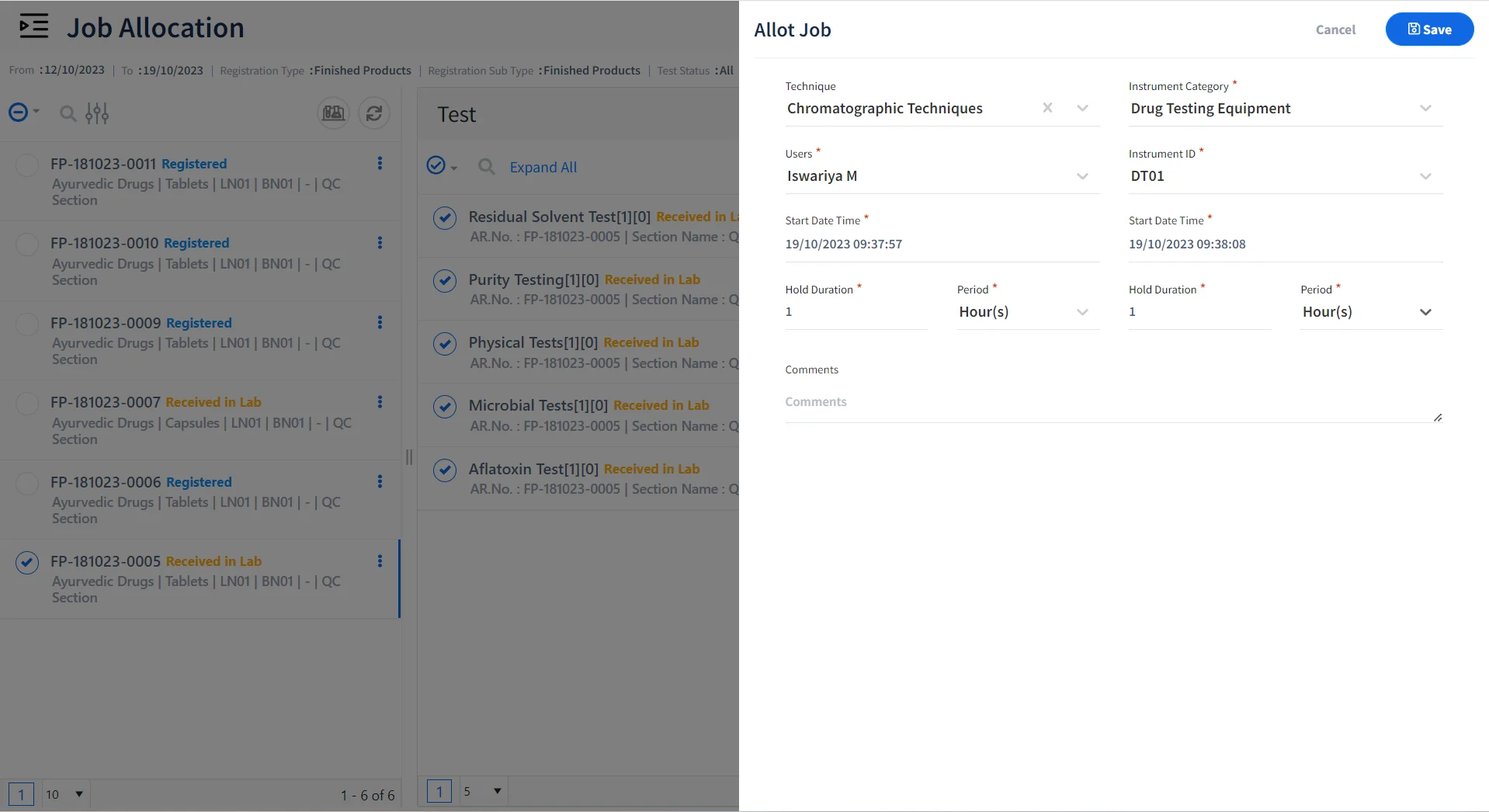
Allocate Jobs to Certified Personnel & Qualified Instruments
- Allocate tests, instruments, and samples to be tested for various personnel.
- Allocate Role based tasks to lab personnel for performing specific tests.
- Ensure that certified personnel are allocated to specific jobs or techniques.
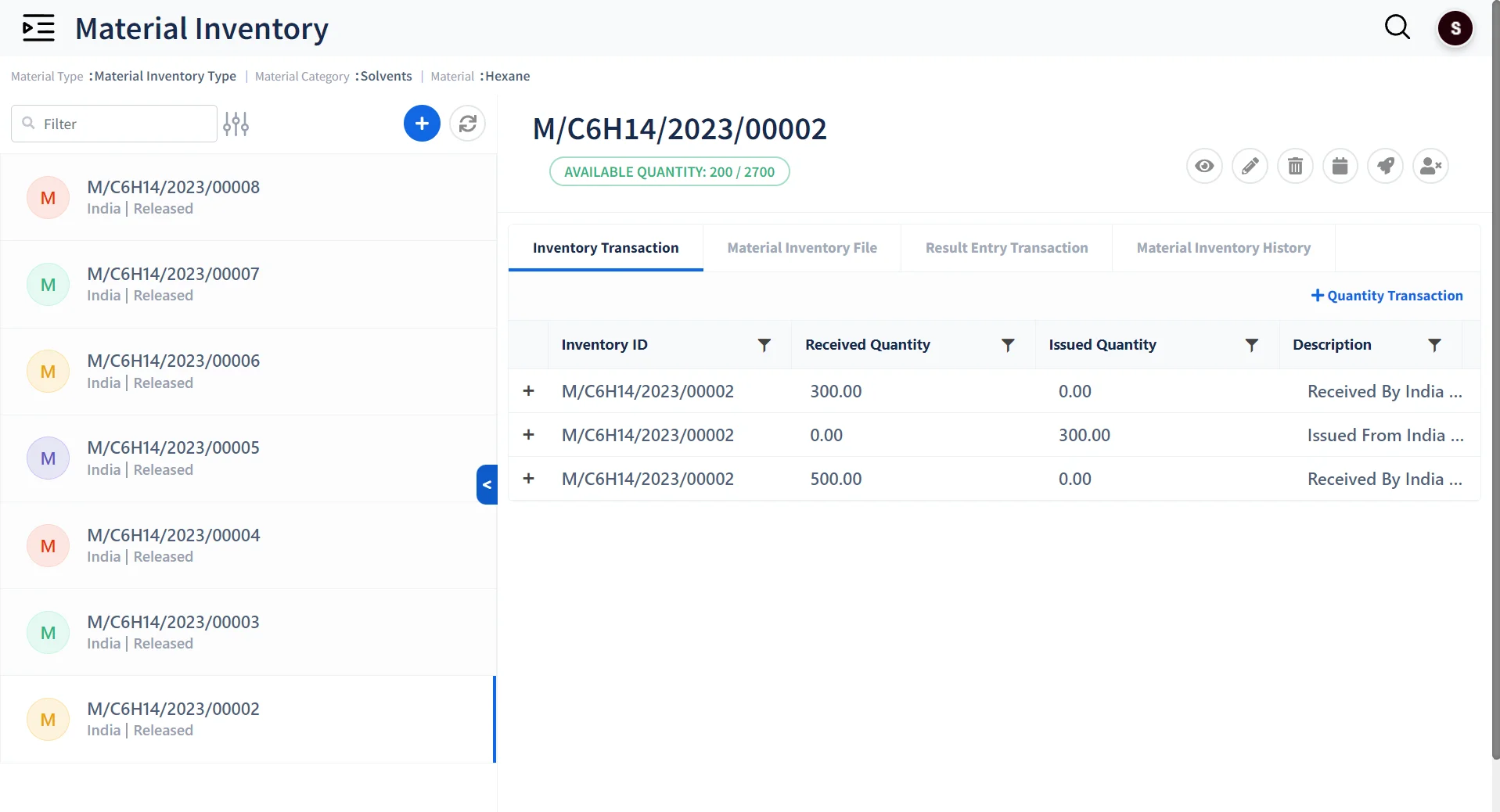
Standardized Inventory Management
- Maintain Multiple Categories of Inventory Material with Barcode labeling.
- Record and track usage of Inventory
- Create Dynamic Storage Structures for Storing Materials
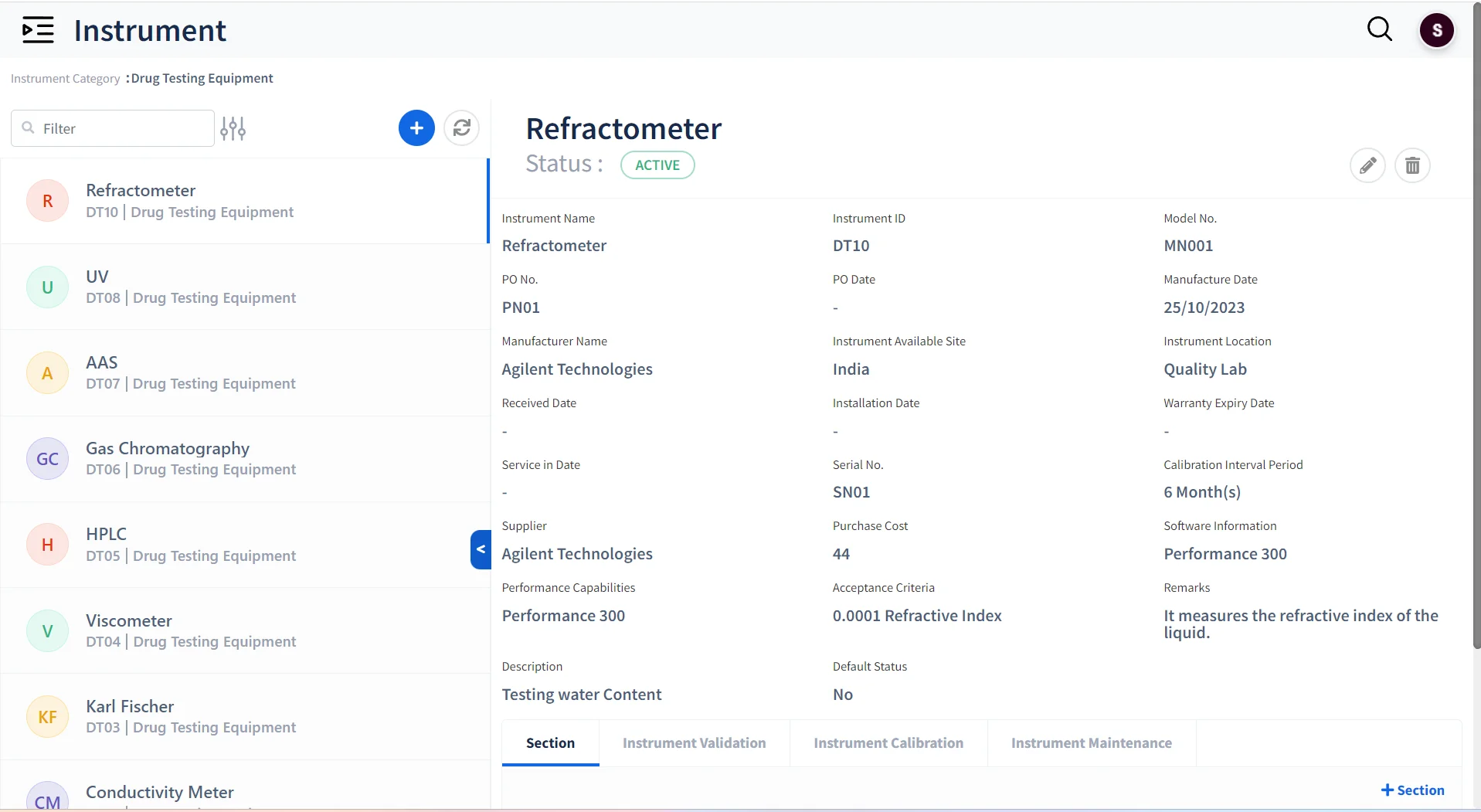
Instrument & Equipment Management
- Maintain all instruments and equipment used across the laboratory
- Keep track of the Validation, Calibration and maintenance of instruments.
- Ensure that only calibrated instruments can be assigned for tests.
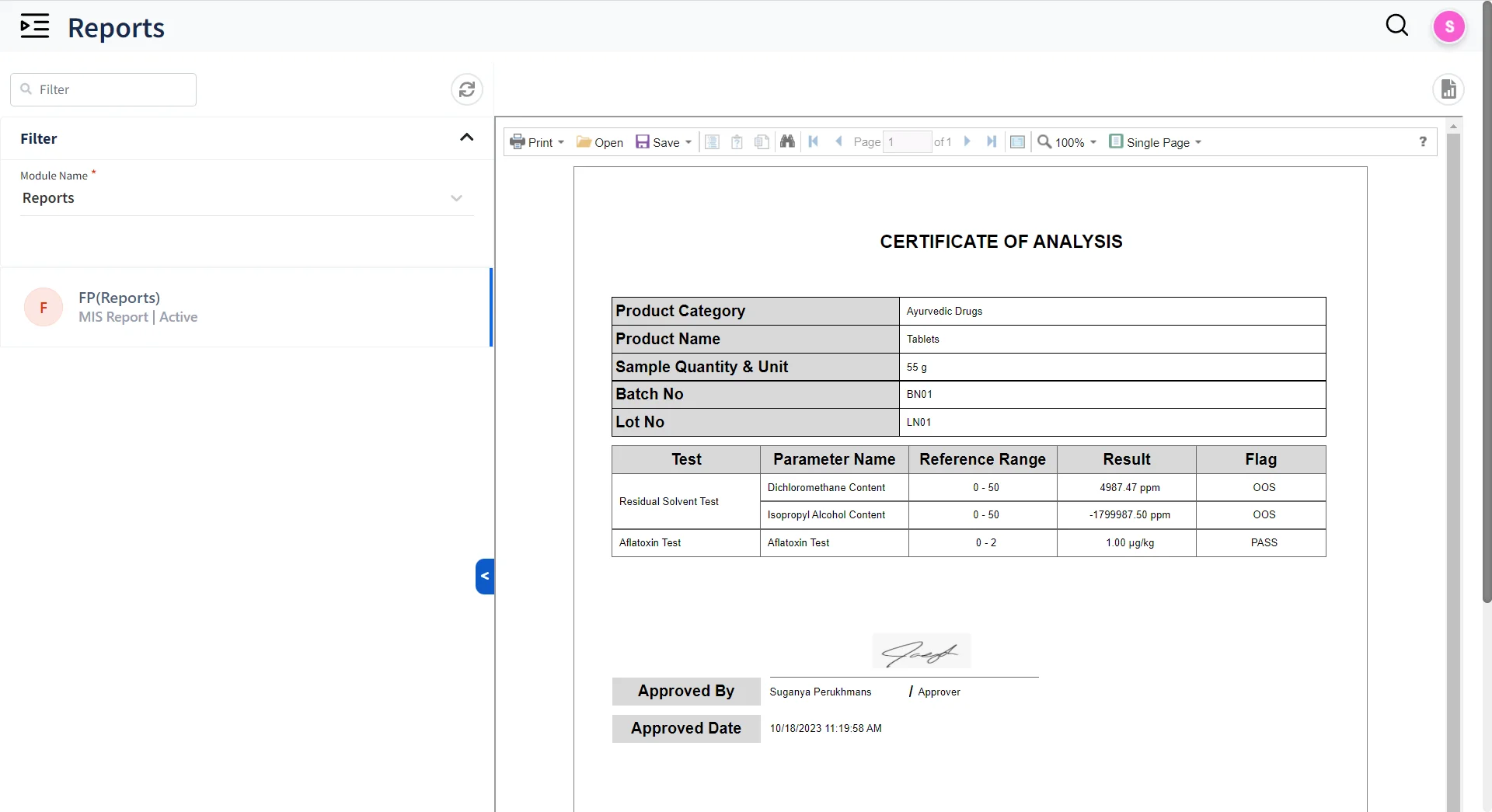
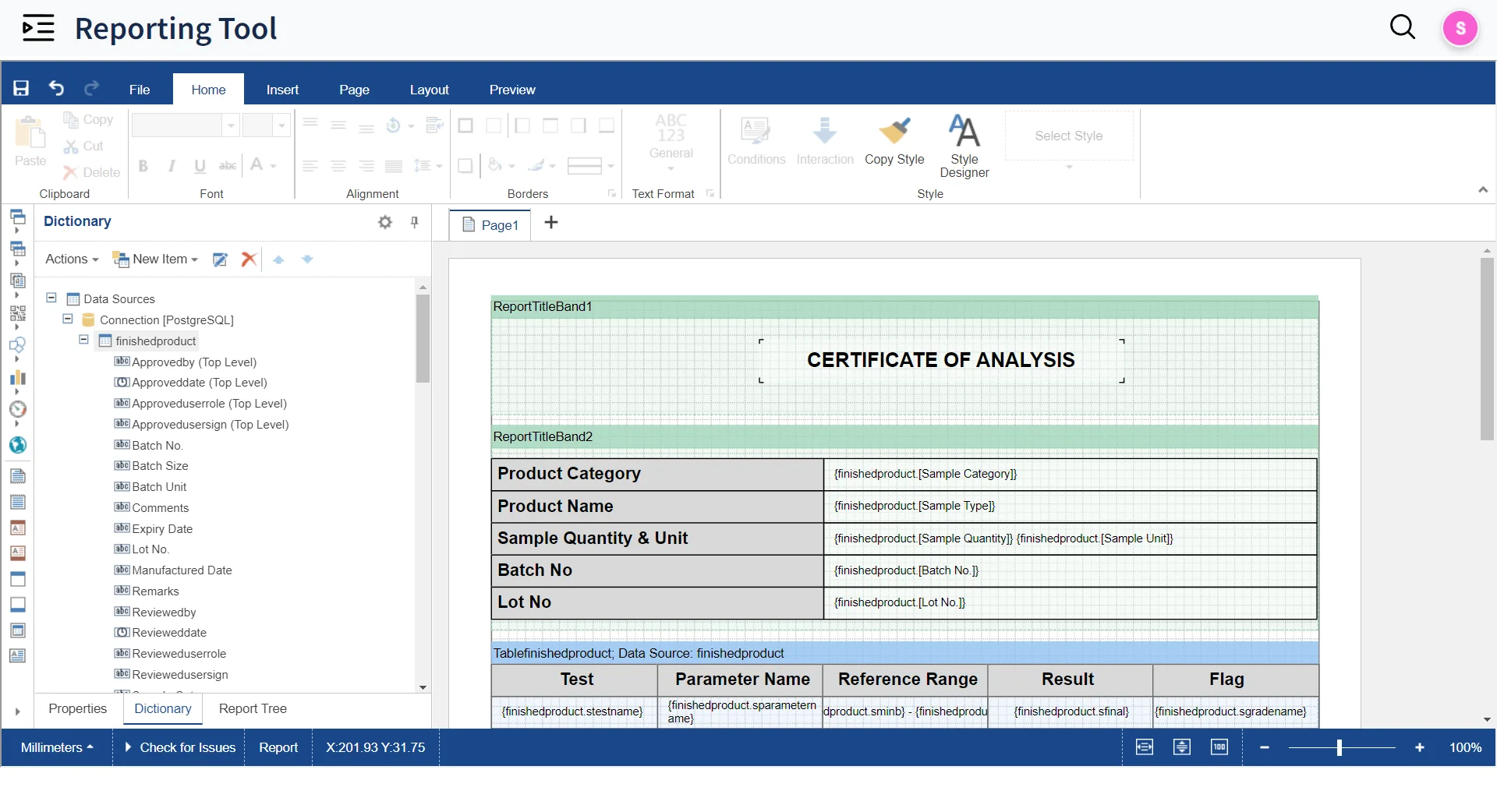
Deliver Accurate Reports on time to your customers & stakeholders
- Built-in Reporting tool to design Templates for Reports
- Query Builder to auto generate SQL queries for Reporting
- Approve and Release reports in seconds with Electronic and digital signatures
Benefits of Qualis LIMS Software

Fully Web Based
Can run using any standard web browser such as Chrome, Firefox, Safari etc

Highly Scalable
Designed for multi-site, multi-department, multi-section, and multi-labs.

21 CFR Part 11 / EudraLex Annex 11 Compliant
High reliability and low cost of compliance due to adherence to Regulatory Compliance standards

GAMP 4
A fully configurable no-code platform enables easy configuration & quicker validation
Over the last two decades, we have built a legacy of partnerships with the following customers.






Dive Deeper into Qualis LIMS

Would you like to learn more about Qualis LIMS?
Email Us: [email protected]