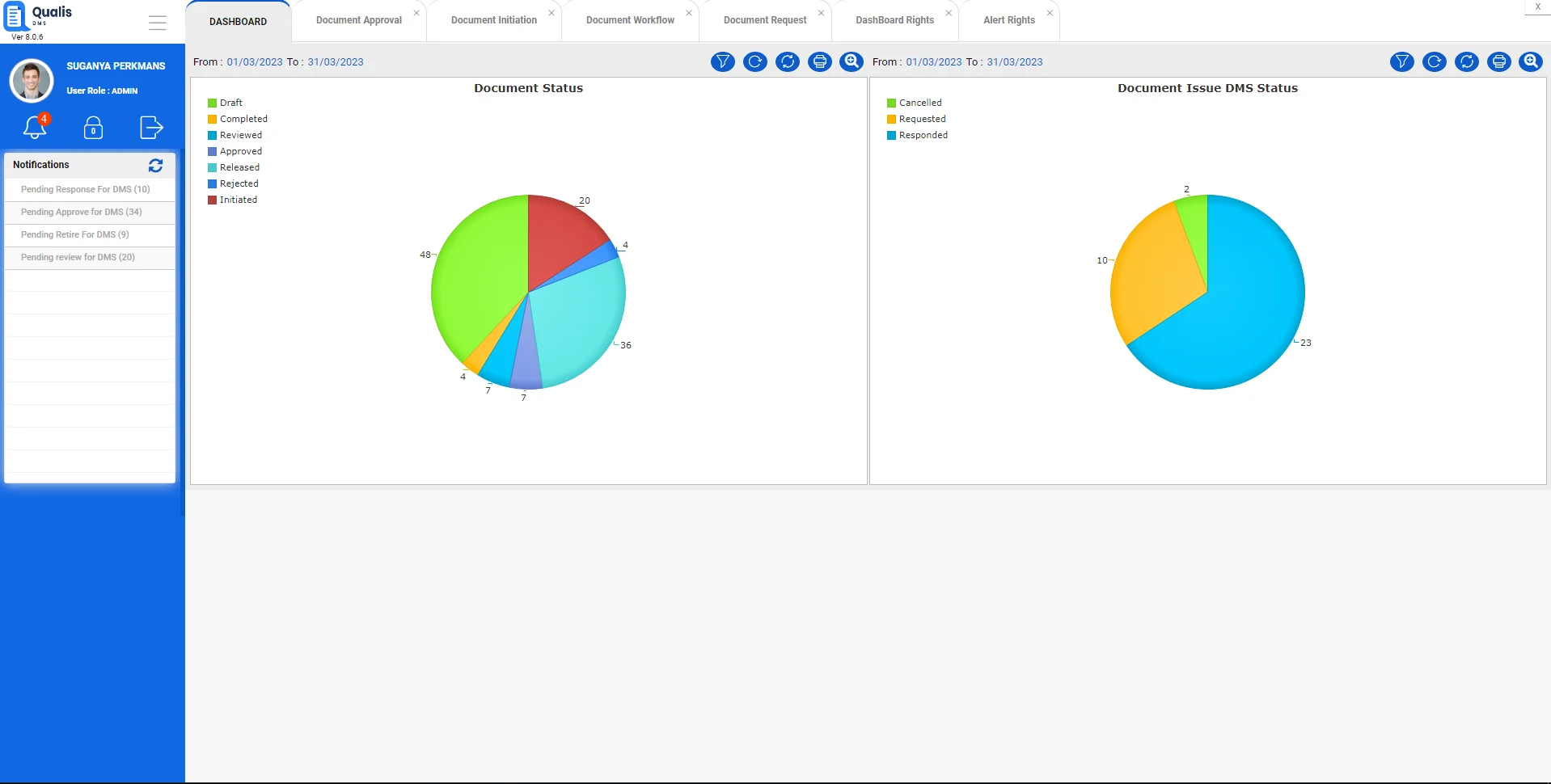
Enterprise Class Document Management & Tracking
Qualis DMS is built to address the growing need or compliant document management in regulated environments.
Centralized Document Access
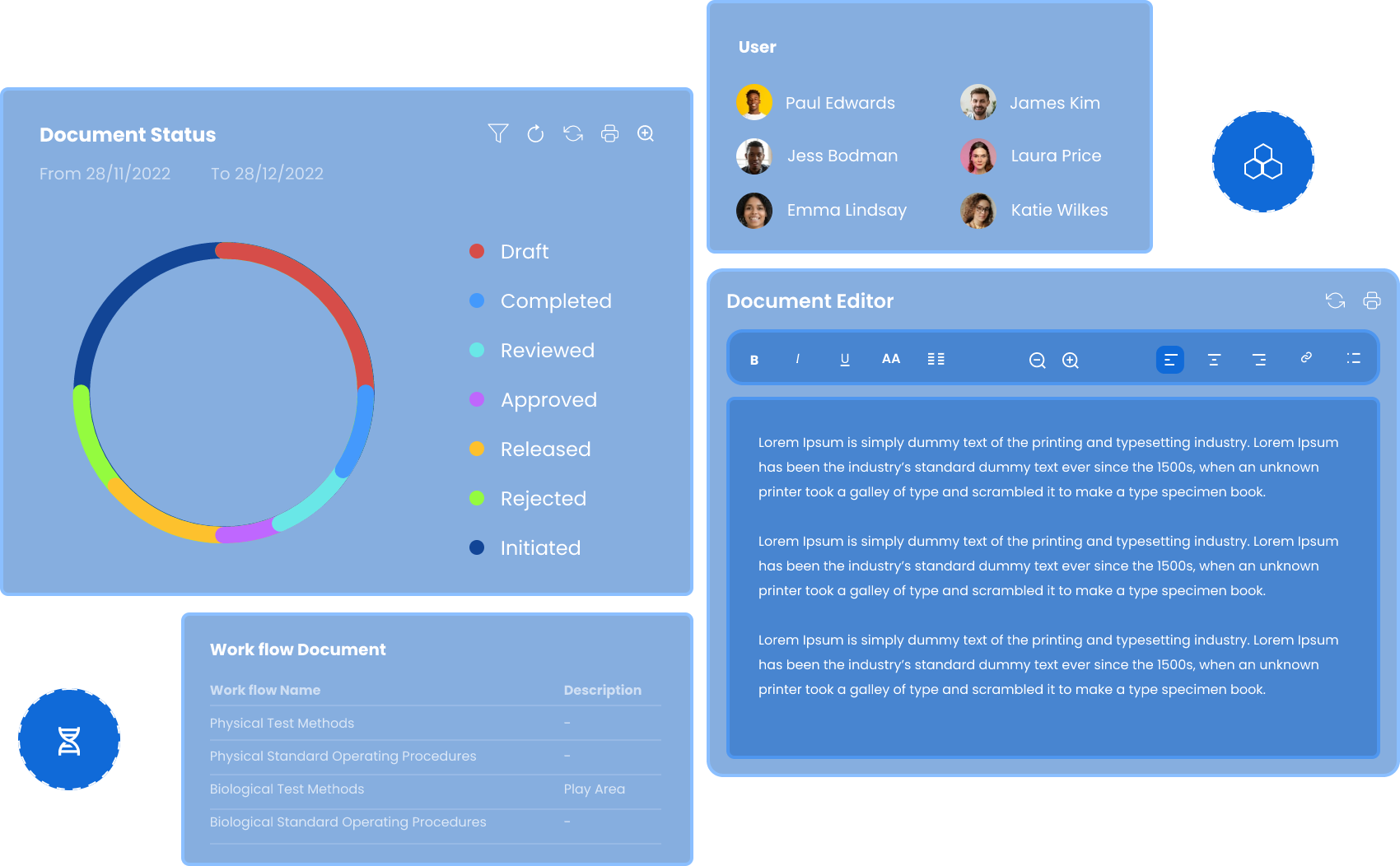
Qualis DMS serves as a single source of truth for document storage, access and control, enabling users to securely store and keep track of electronic documents within the system.
It enables organized, efficient document sharing and collaboration across your local or global enterprise, while ensuring automated version control & approval process for the document throughout its lifecycle.
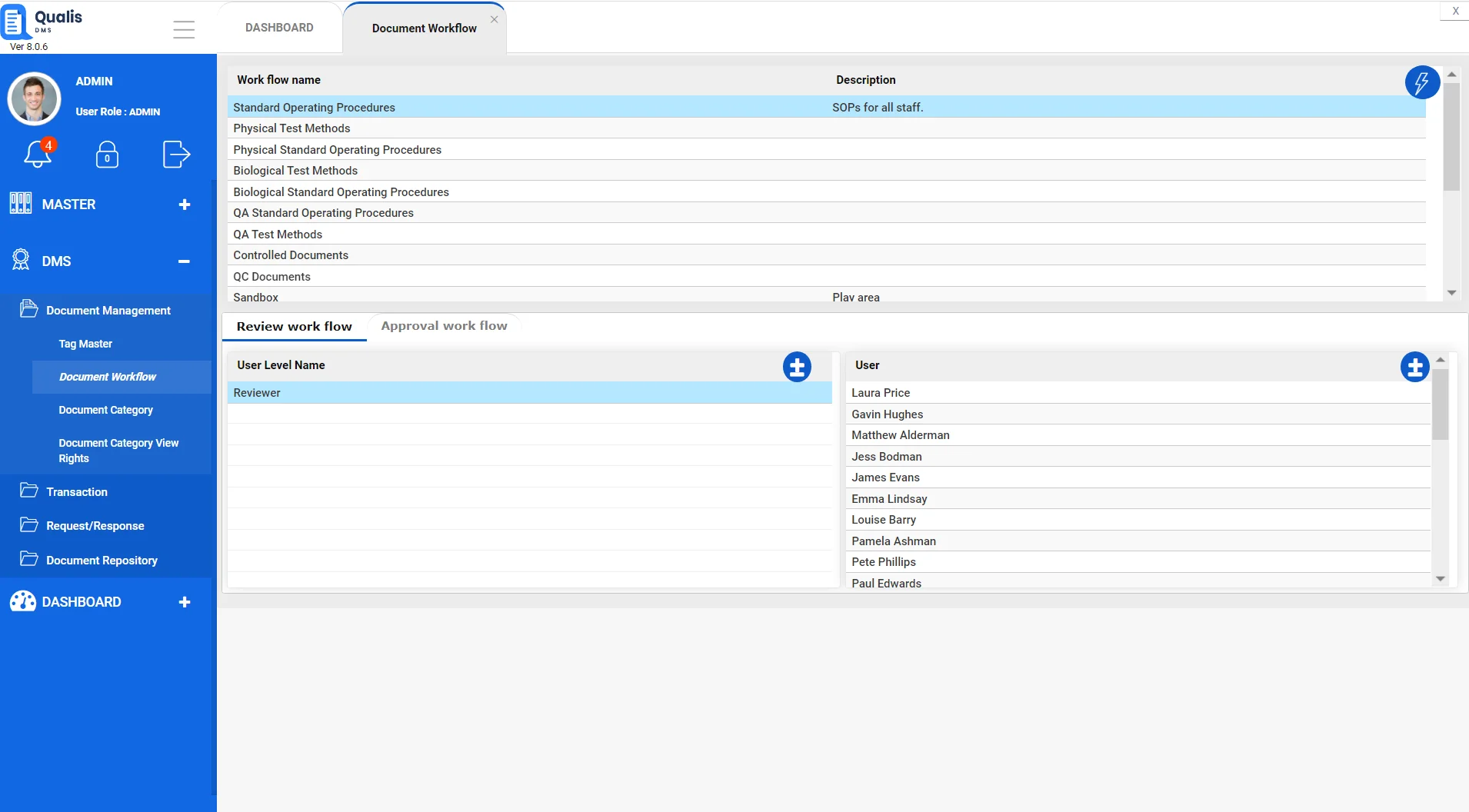
Collaborative Platform for parallel content creation
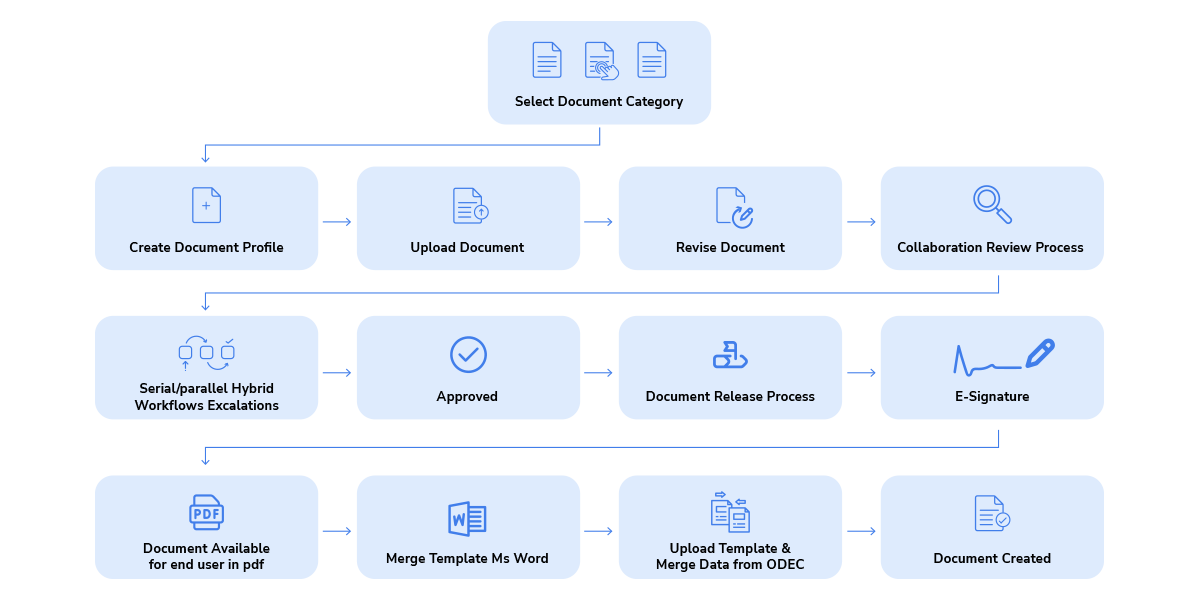
Qualis DMS has a built-in workflow engine which can be configured to meet complex workflows based on different document categories
Work on documents with multiple people within the organisation parallelly for collaborative content creation.
Reconcile and merge content from multiple collaborators into a single document for review and approval using sequential workflows.
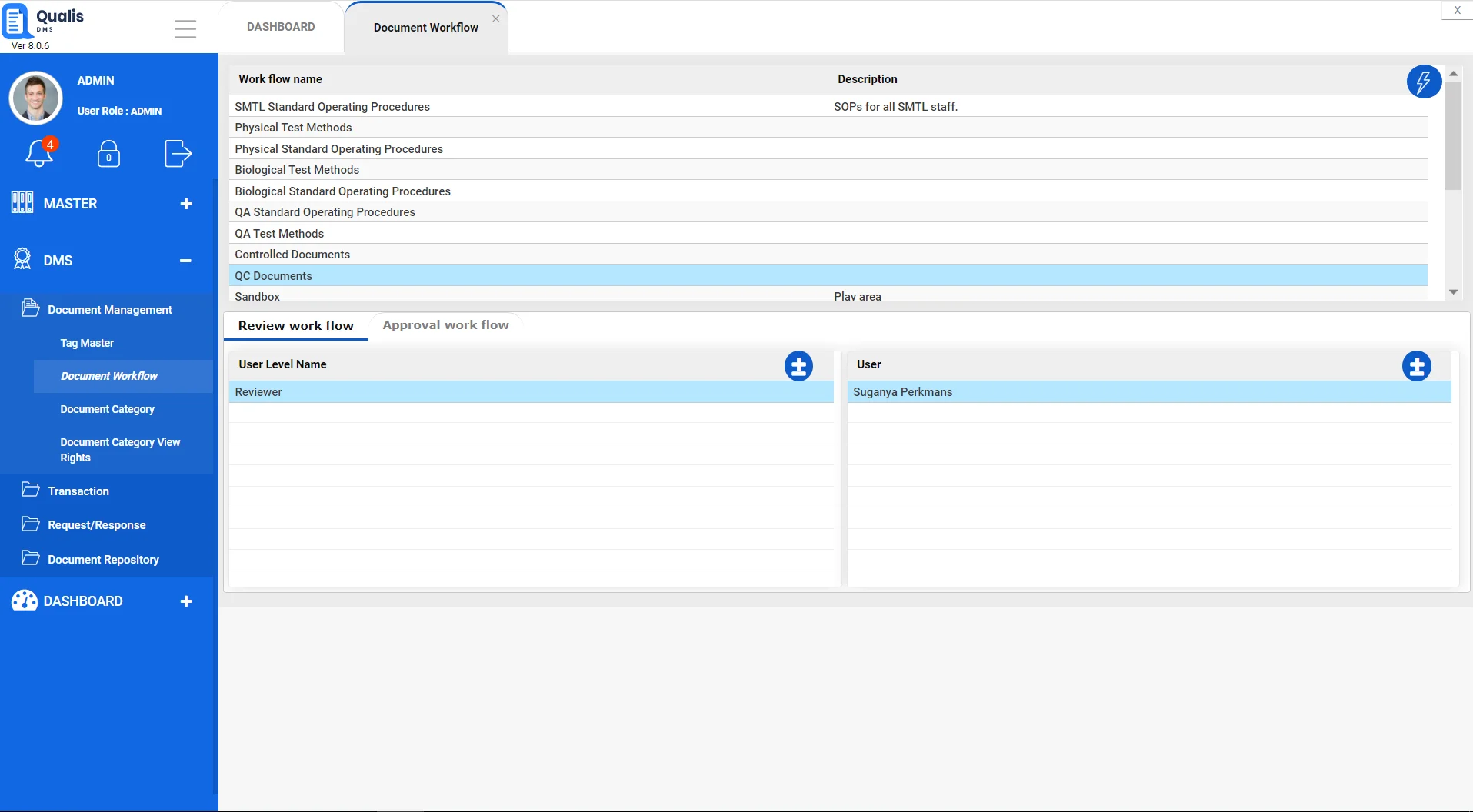
Maintain a contemporaneous record of your data
Qualis DMS Electronic Audit Trail provides a simple solution to understand the complete history of each document. Authorized users can view all details related to the ongoing activity history of a file, including what action was taken, by whom, and the date and time the action occurred.
- Maintain a complete record of every action that takes place to a file throughout its lifecycle.
- Streamline regulatory compliance with industry quality standards and regulations.
- Protect your organization in legal situations with a proven record of the actions executed on a given document.
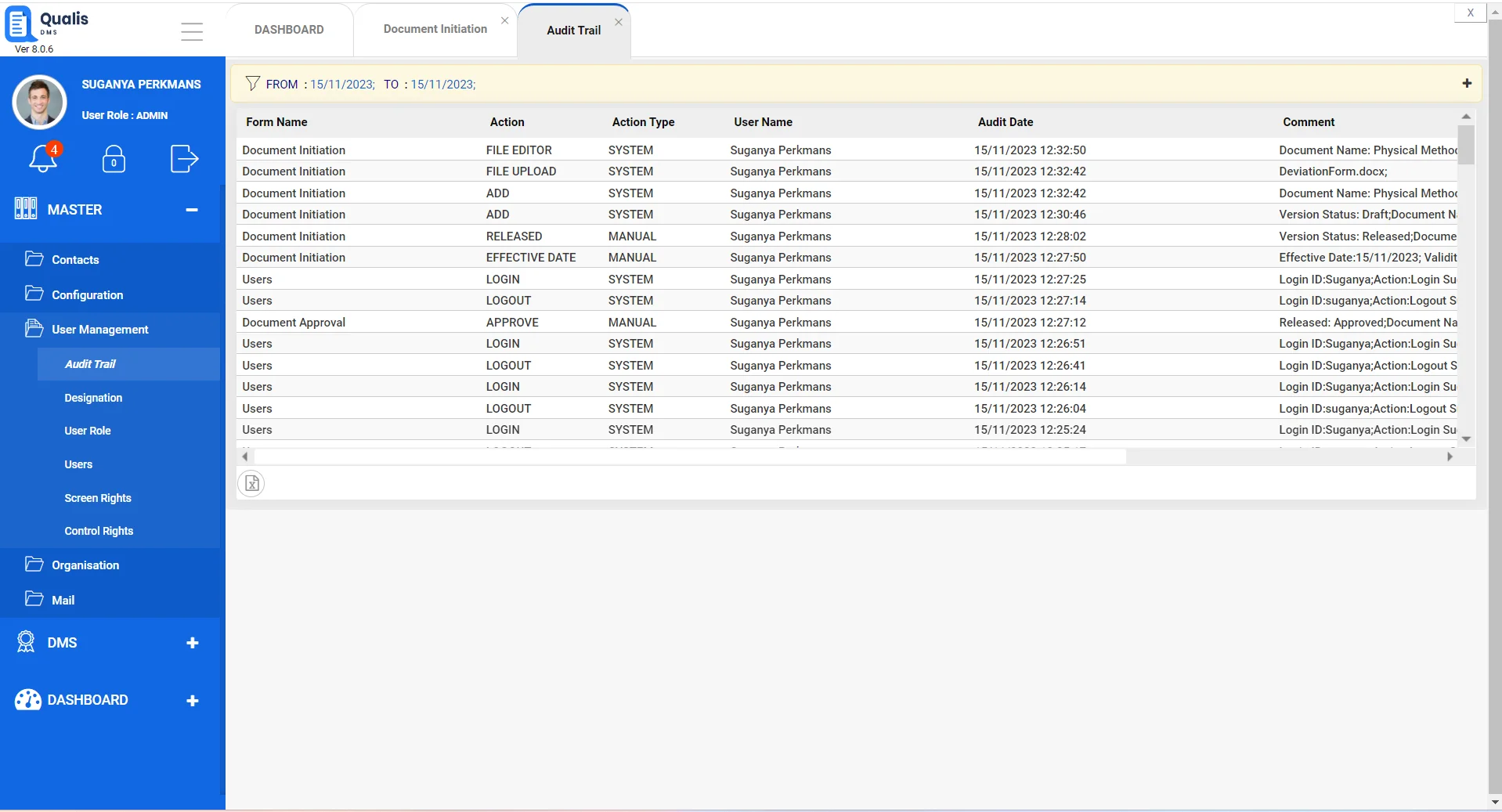
Ensure access to the latest version of documents
Qualis DMS serves as your central point for document access and automatically ensures users always access the latest version of documents. Qualis DMS also saves previous versions and maintains the historical metadata so you can access a snapshot in time as well as the complete document history.
- Ensure all users always have access to the latest version of every document
- Automatically save as many previous versions of your files and associated metadata as you choose
- Ensure an audit trail for each document is maintained throughout its lifecycle
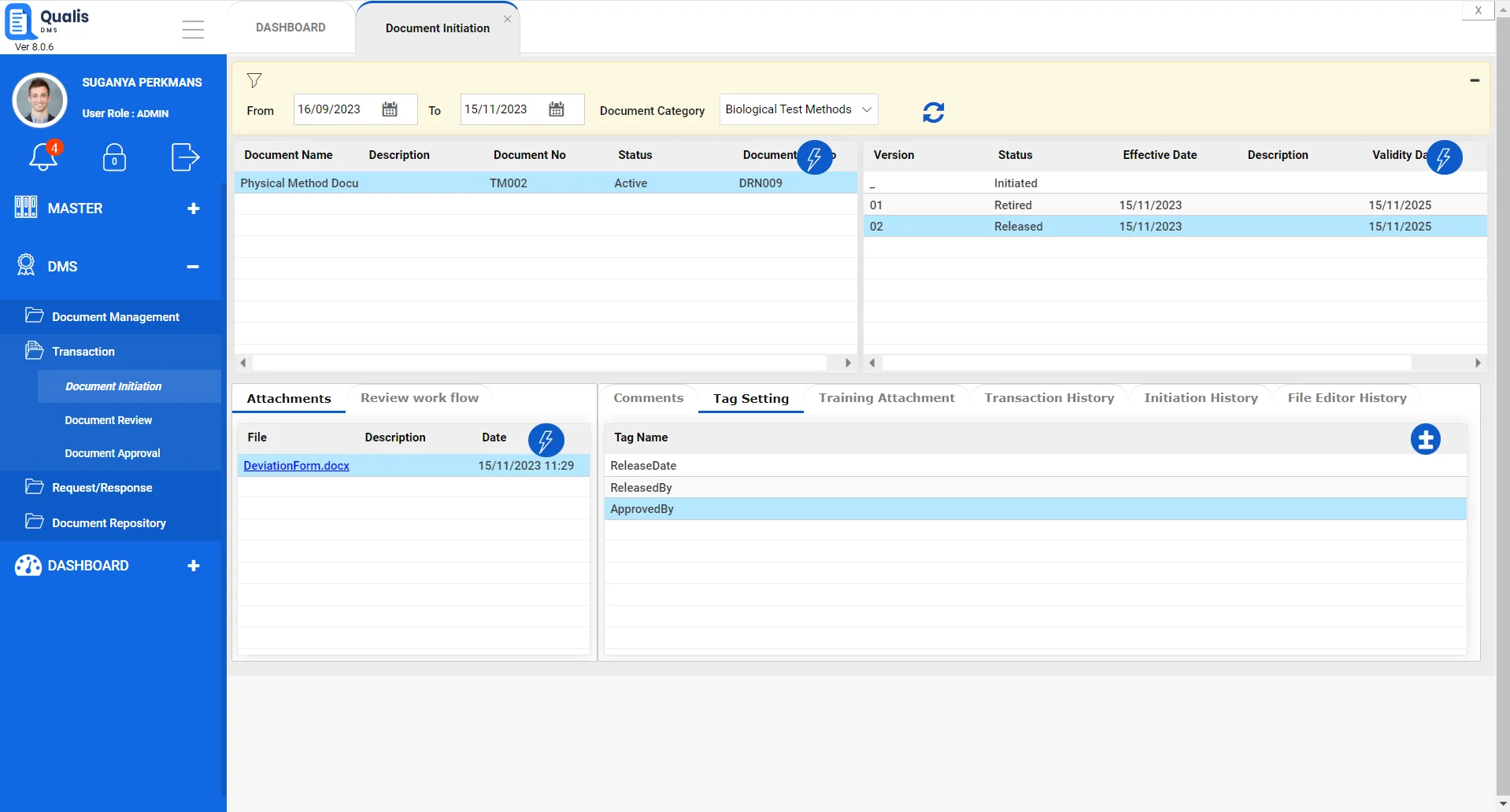
Designed with regulated labs in mind.
Workflow management
Set role-based privileges to users for reviewing & approving documents, with support for e-signatures. Create different kinds of workflows such as serial & parallel hybrid workflows, for each document type.
Version control
Automatically version changes made to documents, enabling traceability throughout the document lifecycle. Comprehensive audit trails help you identify what changes were made and when and by whom.
Controlled access and quick search
Create user roles & provide role based privileges to view & edit documents. Powerful search capabilities enable you to instantly retrieve documents you are looking for.
Discover how Qualis DMS makes your document management compliant
Implement best practices for document control & management in your organization
Qualis DMS ensures that all documents are up-to-date, accurate, and easily accessible to authorized personnel. This results in improved compliance, reduced risk, and increased efficiency in document management processes.
Ensure Traceability
Track all changes made within documents with complete version and release control
Streamlined Workflow
Review and approve documents in a seamless, defined workflow within the system
Access Control
Set user role-based access to documents for increased security and compliance
Secure your digital assets, & improve regulatory compliance
Qualis DMS offers robust security features ensuring that only authorized personnel can access sensitive data. Organisations can safeguard their digital assets, achieve regulatory compliance, and mitigate risks associated with non-compliance.
Password Policy Control
Configurable password policy for user authentication
Comprehensive Audit History
Date and time-stamped audit trails
Adhere to Compliance Requirements
Electronic records protection & E-signature support - 21 CFR Part 11 compliant
Automate Form Filling, Issuance and Printing
Achieve contemporaneous recording of information and control over the usage of the paper-based system while adhering to cGMP data integrity guidelines from the US FDA and MHRA.
Autofill Key Details
Autofill header information such as product name, batch number, lot number, issuance date and time, etc electronically before issuance.
Centralized Document Repository
Create a single source of truth available electronically and automatically and maintain the integrity of the document.
Controlled Document Printing
Control the number of copies of the document that are allowed to be printed.
Quick Releasing & Printing for controlled distribution
Qualis DMS enables quick release and printing of documents to hard copies.
On-Demand Publishing
Print or publish any selection of documents on-demand
Collaborative Document Sharing
Publish to Qualis DMS Library or a network folder for collaboration
Version-Controlled Publishing
Ensure your output is generated from the correct file version
Controlled Print Management
Setup controlled access & approvals for printing and reprinting activities
Benefits of switching to Qualis DMS
Paperless system
The high degree of standardization-is due to the ability to access from any modern web-browser. Reduce dependency on paper-based systems & physical storage.
Low compliance cost
Reduced cost of compliance due to controlled role-based user access, audit trail and easy document tracking & retrieval.
Error-free data handling
Improved reliability & error-free document handling due to automation and workflow-enabled operations.
Improved collaboration
Improved collaboration due to the central storage of documents acting as single source of truth.
Adopted by Leading Pharma and Life Sciences Organisations.




Make your organization’s document management compliant today
Email Us: [email protected]