The realm of cell and gene therapy is witnessing unprecedented advancements, with researchers striving to unlock personalized therapeutic solutions. As the intricacies of this domain grow, the need for robust laboratory informatics becomes paramount.
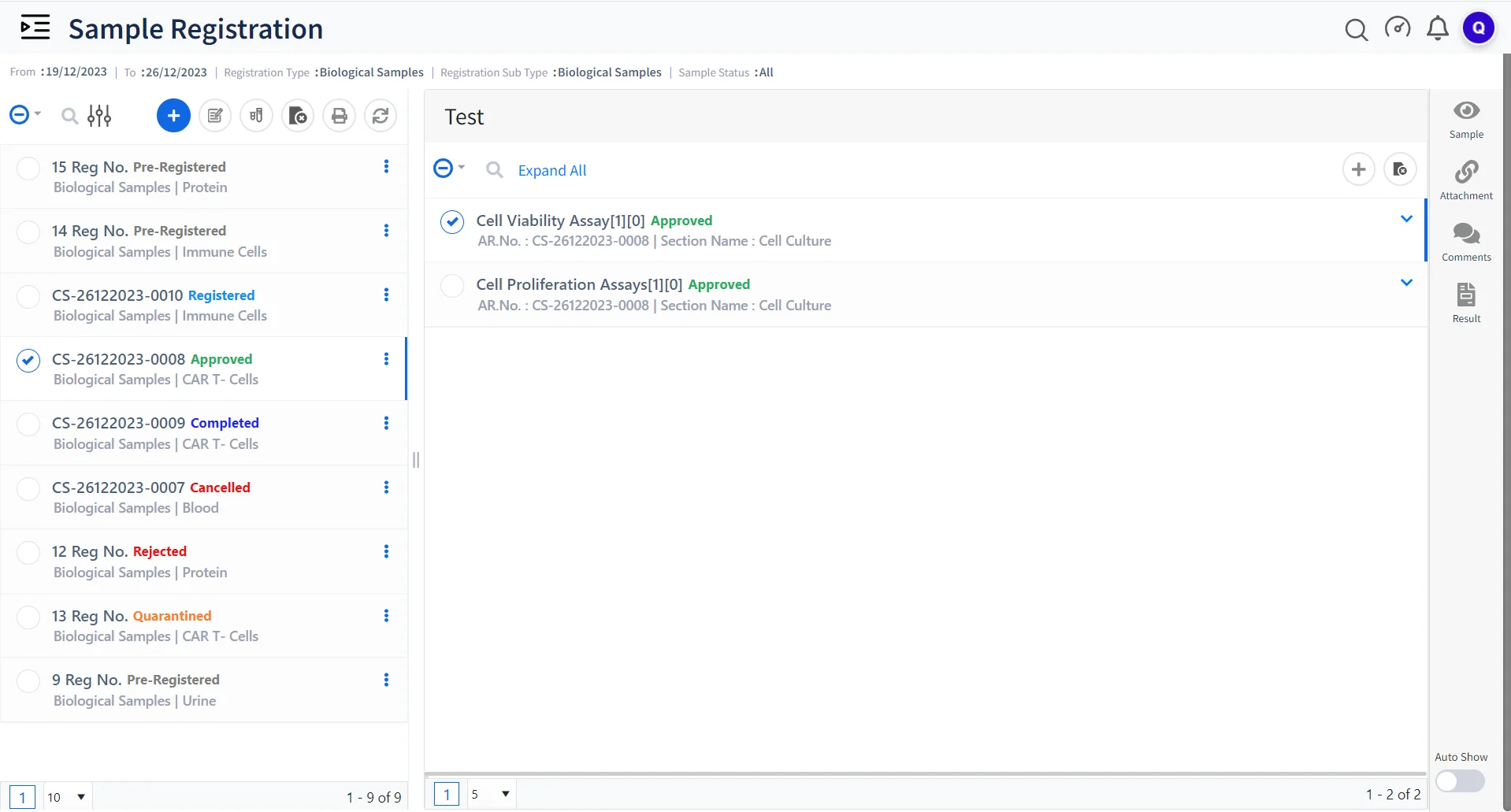
Qualis LIMS for Advanced Sample Management
Sample Tracking
Efficiently track and manage various samples such as CAR T-cells, engineered immune cells, proteins, etc .
Batch Processing and Analysis
Process multiple batches of samples simultaneously, streamlining the analysis and ensuring consistent results across batches.
Data Integrity and Compliance
Ensure that every data point, from sample collection to analysis, adheres to the highest standards of accuracy and regulatory compliance.
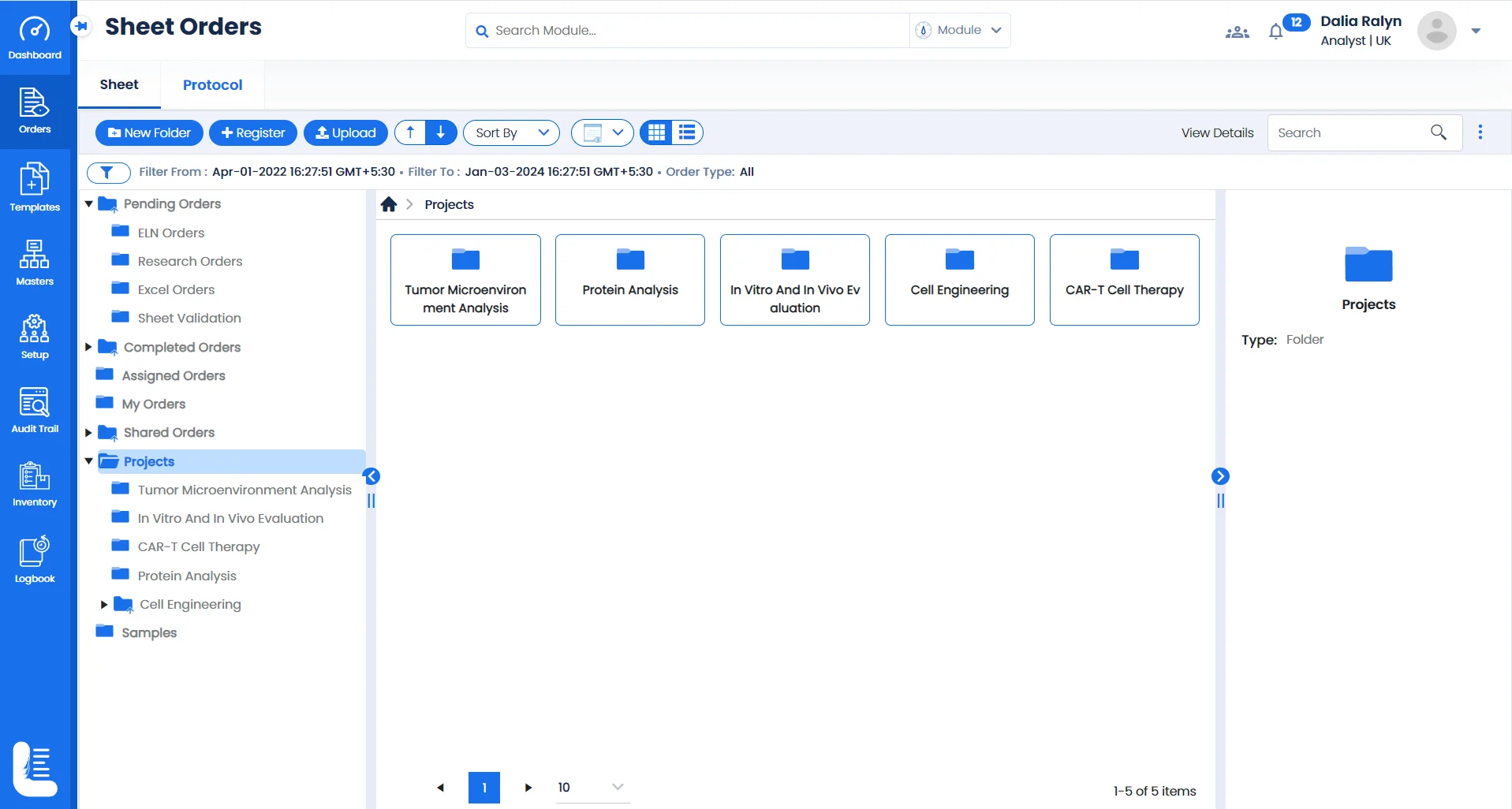
Streamlining Experiments & Projects with Logilab ELN
Sample-Centric Project Management
Utilise a configurable folder based interface to create & manage multiple projects based on tests conducted for different samples such as CAR T-cells, engineered immune cells, protein samples etc.
Focused research studies
Create & manage projects tailored to each area of study whether you're delving into genetic studies , studying cell differentiation, or exploring the potential of viral vectors.
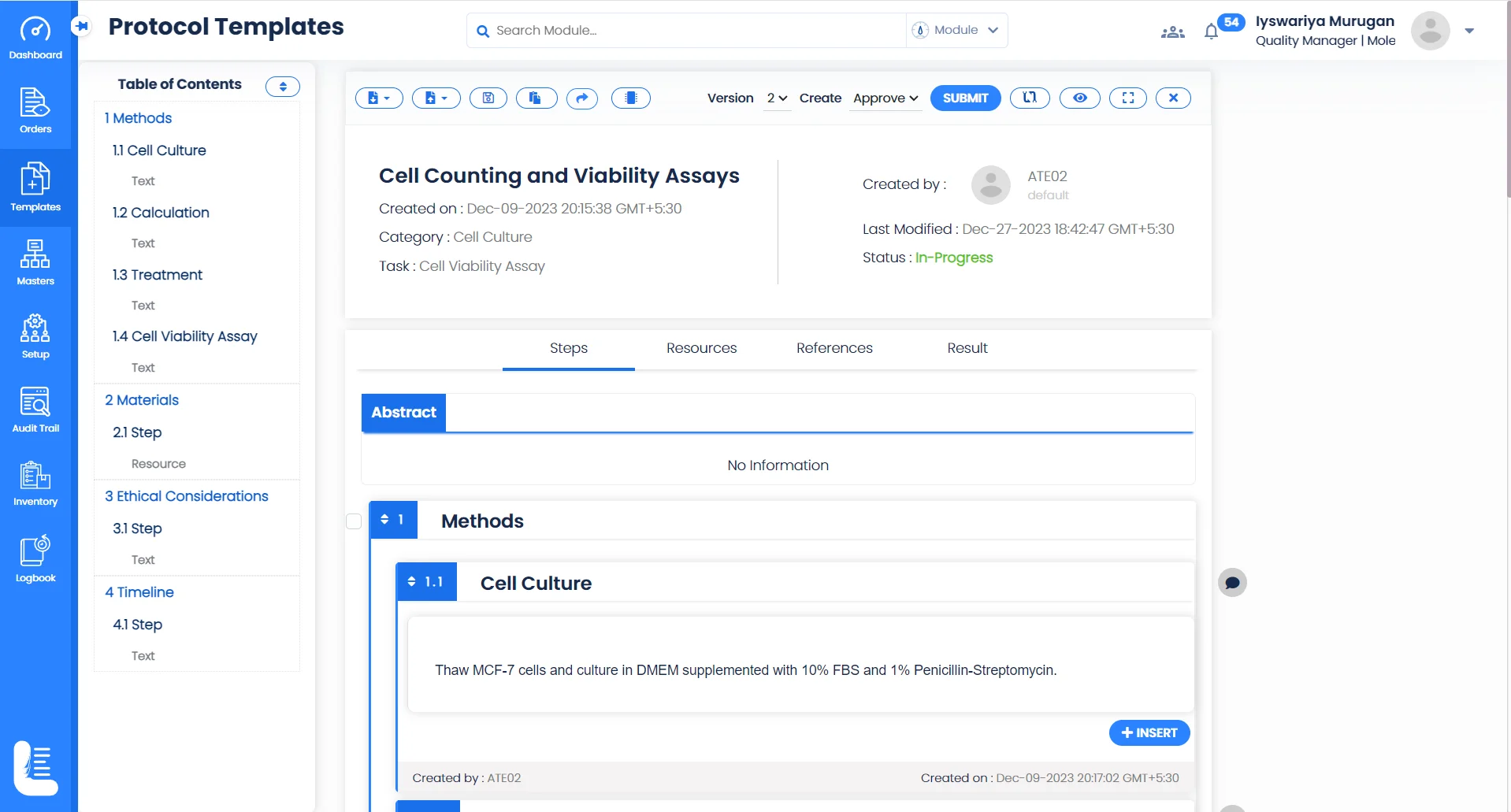
Perform Collaborative Research
Collaborative Research
Logilab ELN facilitates real-time collaboration, allowing researchers to share insights, data, and breakthroughs on projects, fostering a unified approach for collaborative research.
IP Safeguarding
Protect your ground-breaking methodologies and techniques with state-of-the-art security features, ensuring your research remains proprietary.
Harness Your Instrument Data
Seamless Data Extraction
Interface with a wide range of laboratory instruments using Logilab SDMS, ensuring that data is extracted in real-time and without manual intervention. This minimizes errors and ensures that researchers have access to the most accurate data.
Seamless Integration
Logilab SDMS seamlessly integrates with Logilab ELN and Qualis LIMS allowing researchers to extract and use instrument data within their experiments. This ensures that the entire research workflow, from data capture to analysis, is streamlined and efficient.
Revolutionizing Cell and Gene Therapy Research with Logilab ELN and SDMS
With tools like Logilab ELN and Logilab SDMS, researchers in cell and gene therapy are equipped to drive innovation, ensuring that every sample, experiment, and data point is managed with precision and efficiency.

FAQs
Adopting Qualis LIMS can be cost-effective in the long term, despite the initial investment.
Qualis LIMS streamlines complex processes such as testing & managing raw materials, tracking samples, and ensuring compliance with stringent regulatory standards.
These efficiencies can lead to significant time savings, reduced errors, and improved data management.
Additionally, as these labs grow and scale, having a robust LIMS in place can be critical to managing increased data volumes and complexity, ultimately contributing to sustained cost-effectiveness and operational scalability.
Logilab ELN is ideal for managing the intricate documentation involved in genetic research. It enables researchers to meticulously record experimental protocols, gene sequencing data, and editing outcomes. The ELN's digital format allows for detailed annotations, easy retrieval of past experiments, and supports compliance with evolving regulatory standards in gene therapy.
Logilab SDMS is adept at managing large datasets typical in cell therapy clinical trials. It efficiently stores, organizes, and secures trial data, including patient responses, genomic data, and treatment outcomes. Its robust data management capabilities facilitate comprehensive analysis and reporting, which are essential for clinical study evaluations and regulatory submissions.
Qualis LIMS is CFR Part 11 compliant, featuring audit trails, electronic signatures, and user access controls. Generate compliant reports with ease, manage lot release procedures, and ensure adherence to regulatory requirements.