Chemical manufacturers maintain a steadfast commitment to ensuring the highest quality products through rigorous production and process controls. This requires vigilant monitoring of samples throughout various stages, including raw materials, in-process samples, finished products, and the materials employed in the synthesis of diverse chemicals. Consequently, chemical plants remain active in their continual surveillance and analysis of these crucial components to safeguard the quality of their final products.
The traditional methods of maintaining quality control and monitoring samples involve manual testing & monitoring, which can be time-consuming and prone to errors. With the increasing complexity of modern chemical formulations, this process has become more complicated, with several instruments and inventories used in the manufacturing process.
Our innovative solutions enable labs to execute and monitor tests digitally, maintaining the highest standards of quality and compliance

Manage Samples, Tests, & Standard Operating Procedures in a secure central database
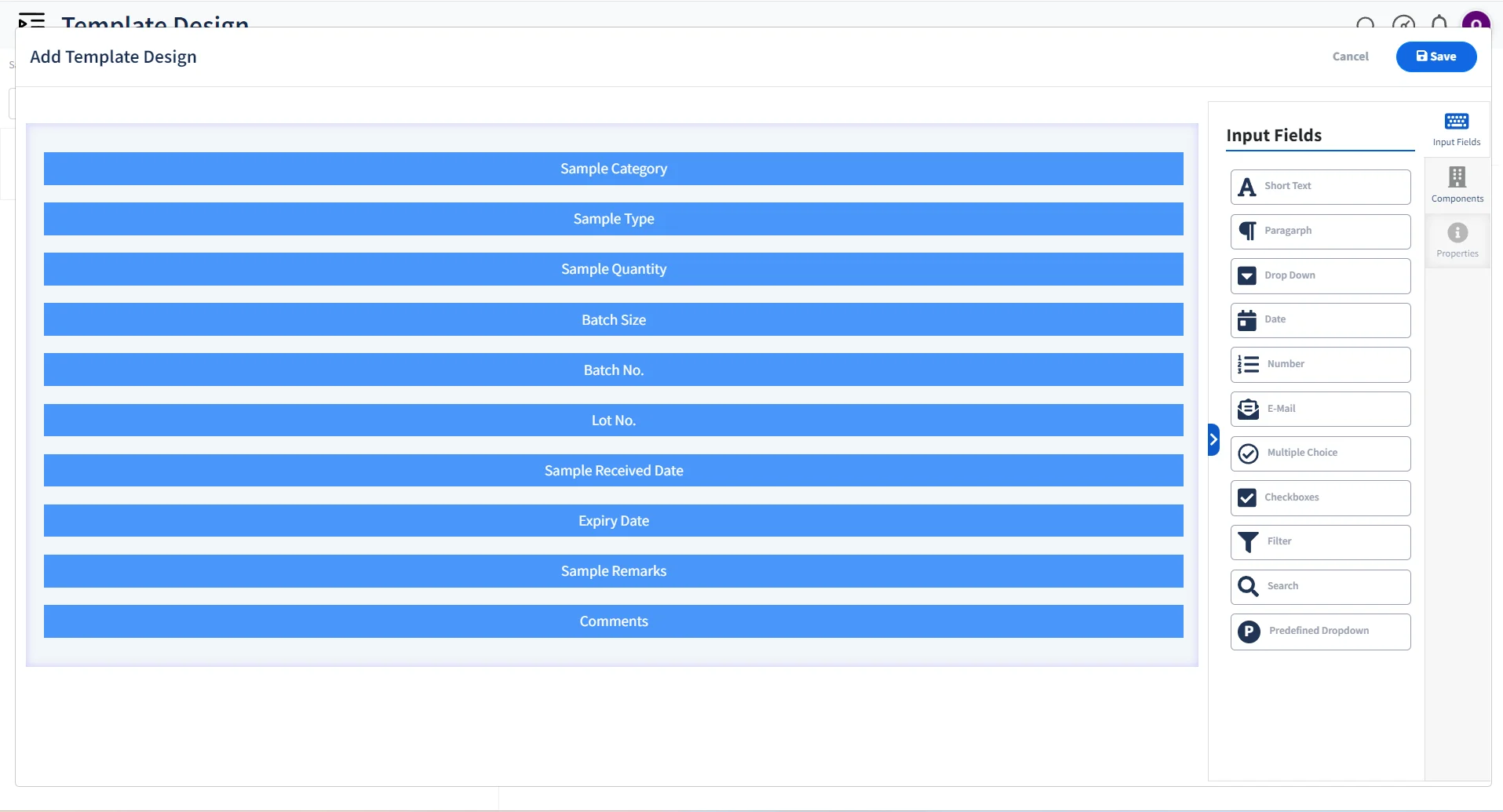
Configurable Sample Registration
User-friendly & customizable template-based sample registration process.
Prepare Study Plans
Quality control test planning for continuous & batch manufacturing of chemicals.
Generate Barcodes
Barcode labeling & printing capability.
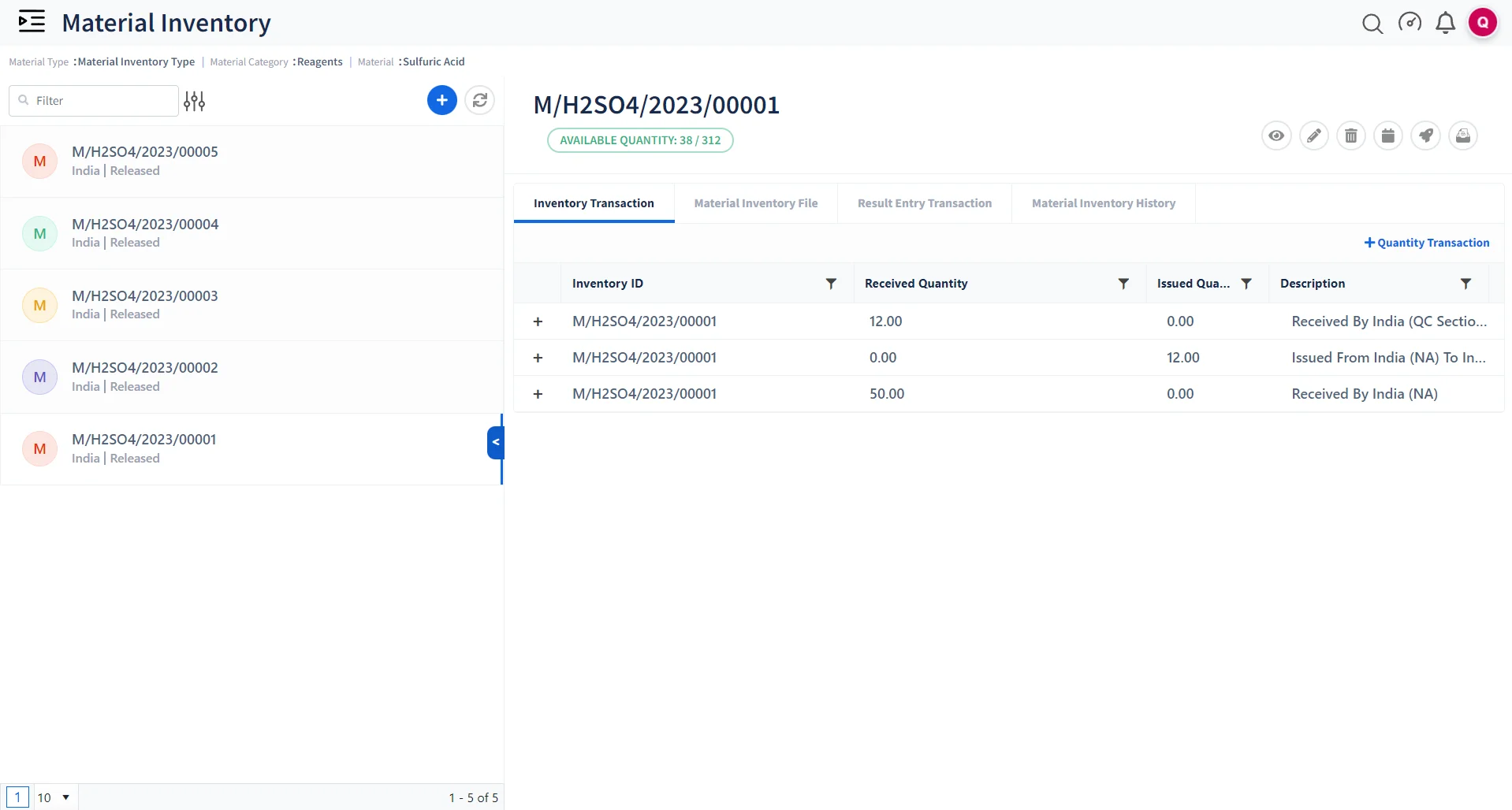
Streamline Your Inventory Management
Track Inventory Usage
Inventory management to keep track of lab materials & consumables used in the manufacturing of chemicals.
Manage OOS Results
Out-of-specification reporting with configurable review & approval workflows.
Track Instrument Usage
Equipment and instrument management with instrument calibration & maintenance alerts.
FAQs
Qualis LIMS offers robust chemical inventory management capabilities that are crucial for the chemical industry. It tracks chemicals from receipt to disposal, monitors stock levels, manages safety data sheets, and ensures regulatory compliance with chemical handling and storage. This comprehensive approach helps manage the unique challenges of handling a wide variety of chemicals, including hazardous and controlled substances.
Logilab ELN is particularly effective in streamlining formula development and experimentation in chemical research. It allows chemists to digitally record experiments, track formula iterations, and analyze data in real-time. The ELN facilitates collaboration, maintains a detailed and searchable record of research activities, and helps in the quick adaptation of formulas, thus accelerating product development cycles.
Logilab SDMS is adept at handling and organizing the complex data generated from spectroscopy and chromatography instruments. It centralized data storage, supports various data formats, and allows for advanced data analysis and visualization. This is essential in the chemical industry for maintaining the integrity of analytical data and ensuring accurate results.
Yes, Logilab SDMS supports long-term data retention and retrieval, which is crucial for studies on chemical stability and shelf-life. It offers secure, long-term storage of experimental data, with robust backup and recovery processes. This ensures that historical data remains accessible and intact for future analysis, essential for ongoing stability and shelf-life studies in the chemical industry.